Support for the international vaccine development partnership the Coalition for Epidemic Preparedness Innovations, which has advanced eight approved COVID-19 vaccines and is furthering candidates against other epidemic and pandemic threats.
US agency series: Global health R&D across the US government
This six-part fact sheet series examines the contributions of US government agencies to advancing global health R&D. The series includes the US Agency for International Development, National Institutes of Health, Centers for Disease Control and Prevention, Biomedical Advanced Research and Development Authority, Department of Defense, and Food and Drug Administration.
Global Health R&D at the US Agency for International Development
 What does USAID do for global health R&D?
What does USAID do for global health R&D?
The US Agency for International Development (USAID) supports the development, introduction, and scale-up of urgently needed drugs, vaccines, diagnostics, and other technologies to address unmet health needs of people in the world’s poorest places. The agency specializes in late-stage clinical research and implementation research to advance new solutions, and its investments also help to strengthen research capacity in partner nations.
 Why is USAID’s role in global health R&D important?
Why is USAID’s role in global health R&D important?
USAID is the only US agency with a mandate to focus on global health and development, which means it is uniquely positioned to support product development and innovation to advance global health. The agency’s deep international footprint, combined with its in-depth understanding of community needs and culture, makes it critical for developing new health tools that are appropriate, affordable, and accessible for widespread uptake in low-resource settings.
 Impact of Investment
Impact of Investment
USAID support has helped advance at least:
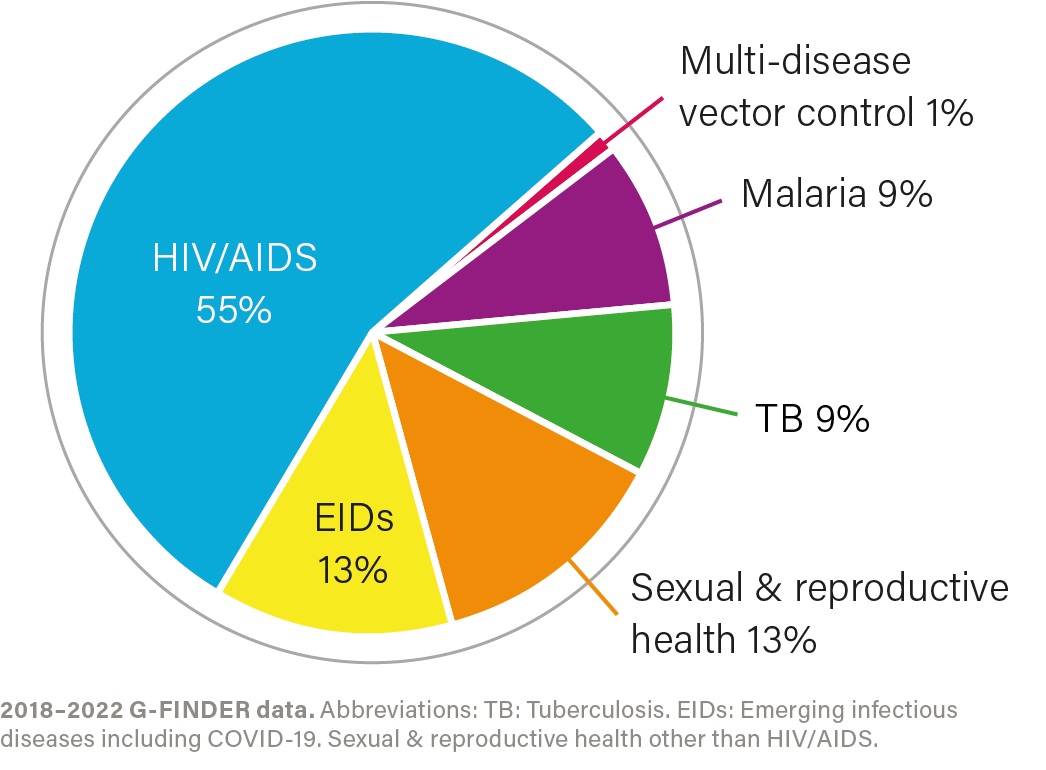
 R&D investment by health area
R&D investment by health area
IMPACT OF INVESTMENT
GLOBAL HEALTH SECURITY
Support for the international vaccine development partnership the Coalition for Epidemic Preparedness Innovations, which has advanced eight approved COVID-19 vaccines and is furthering candidates against other epidemic and pandemic threats.
HIV/AIDS
Development of a woman-controlled microbicide vaginal ring, the first long-acting HIV prevention method to receive a positive regulatory opinion.
MALARIA
Development of a child-friendly malaria medicine, which has been distributed in more than 50 nations and has saved an estimated 926,000 child lives since its introduction in 2009.
TB
Development of child-friendly tuberculosis (TB) medicines, which have been adopted in more than 123 countries that collectively account for more than 75 percent of the world's children reported to have TB.
NEWBORN HEALTH
Adaptation of the antiseptic chlorhexidine for umbilical cord care, which is projected to save the lives of more than 1 million babies by 2030 at a cost of less than $.50 per dose.
MENINGITIS
Development of a low-cost meningitis A vaccine, which, as of 2020, has been delivered to more than 340 million people in 24 countries, virtually eliminating meningitis A wherever it has been used.
Global Health R&D at the National Institutes of Health
 What does NIH do for global health R&D?
What does NIH do for global health R&D?
The National Institutes of Health (NIH) advances basic, applied, and clinical research across a range of global health areas and products. NIH facilitates this research through in-house programs and grants to universities, nonprofits, and other organizations and operates clinical trial networks that serve as the backbone for clinical trials taking place across America and the world.
 Why is NIH’s role in global health R&D important?
Why is NIH’s role in global health R&D important?
NIH is the United States’ leading medical research institution and a respected, world-class scientific powerhouse. Its work to advance research for global infectious diseases, through the National Institute of Allergy and Infectious Diseases; to coordinate crosscutting HIV/AIDS research, through the Office of AIDS Research; and to strengthen international research capacity, through the Fogarty International Center, forms the building blocks for future drugs, vaccines, diagnostics, and other tools that save and improve lives around the world.
 Impact of Investment
Impact of Investment
NIH support has helped advance at least:
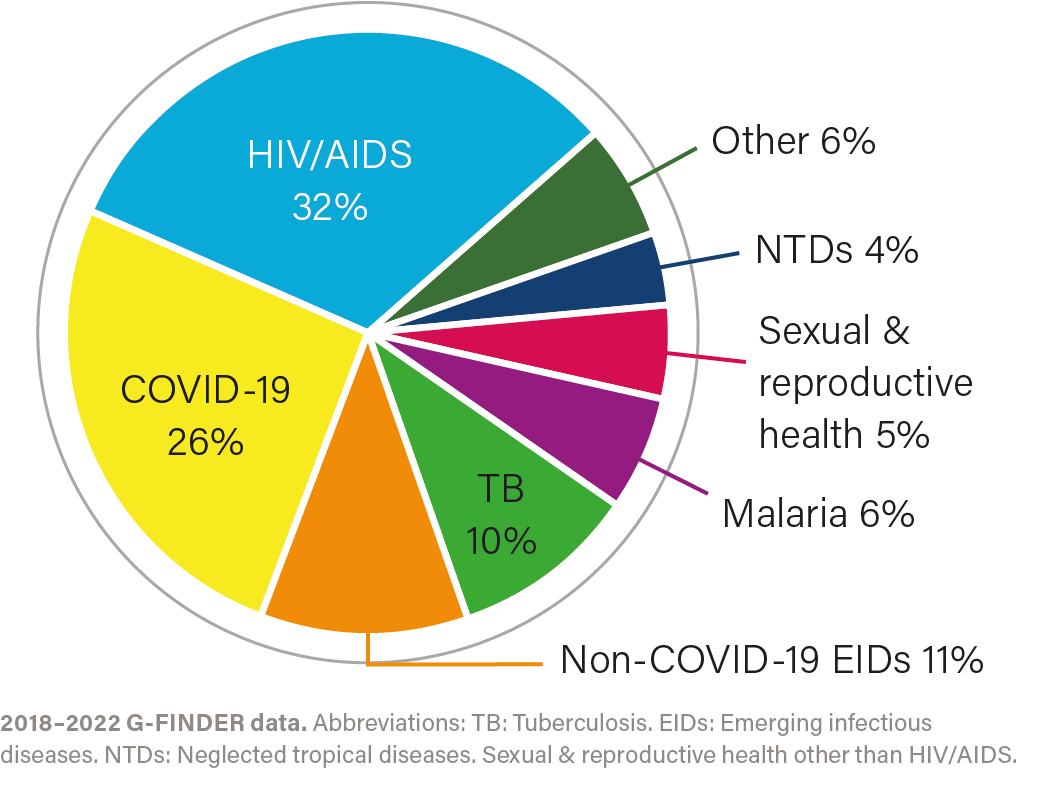
 R&D investment by health area
R&D investment by health area
IMPACT OF INVESTMENT
HIV/AIDS
Development of the first antiretroviral drugs and other subsequent therapies, which have collectively averted an estimated 16.5 million AIDS-related deaths since 2011.
TB
Development of a new drug for drug-resistant tuberculosis (TB), pretomanid, which is part of a regimen that has dramatically improved treatment outcomes and reduced treatment time and could lead to global cost savings of $740 million annually.
NTDs
Development of new tools to combat neglected tropical diseases (NTDs), including two treatments for visceral leishmaniasis and rapid diagnostic tests for Chagas’ disease and river blindness.
DIARRHEAL DISEASE
Creation of two low-cost rotavirus vaccines manufactured in India, ROTAVAC and ROTASIIL, that are now in use in several countries worldwide.
CAPACITY-STRENGTHENING
The Fogarty International Center has provided research training to more than 8,500 US and foreign scientists working in low- and middle-income countries, including alumni who have played vital roles in the Ebola, Zika, COVID-19, and HIV/AIDS responses.
GLOBAL HEALTH SECURITY
Advancement of key discoveries that led to the development of the mRNA vaccine technology that was successfully leveraged for COVID-19 vaccines and is being advanced for other disease threats.
Global Health R&D at the US Centers for Disease Control and Prevention
 What does CDC do for global health R&D?
What does CDC do for global health R&D?
The US Centers for Disease Control and Prevention (CDC) protects people at home and abroad through disease surveillance, rapid outbreak response, and research and development (R&D) of diagnostics, drugs, and other technologies to combat infectious diseases. Not only does CDC’s research advance new diagnostic, prevention, and surveillance technologies, it also evaluates the effectiveness of tools already in use to determine future R&D needs. CDC’s Global Health Center and National Center for Emerging and Zoonotic Infectious Diseases lead much of the agency’s global health R&D work.
 Why is CDC’s role in global health R&D important?
Why is CDC’s role in global health R&D important?
CDC has unique expertise and capacity to detect, track, and contain infectious disease outbreaks and develop the right technologies to advance these efforts. CDC’s work is critical to protecting Americans and people around the world from emerging epidemics, as well as monitoring the impact of current tools and global health programs to maximize future investments.
 Impact of Investment
Impact of Investment
CDC support has helped advance at least:
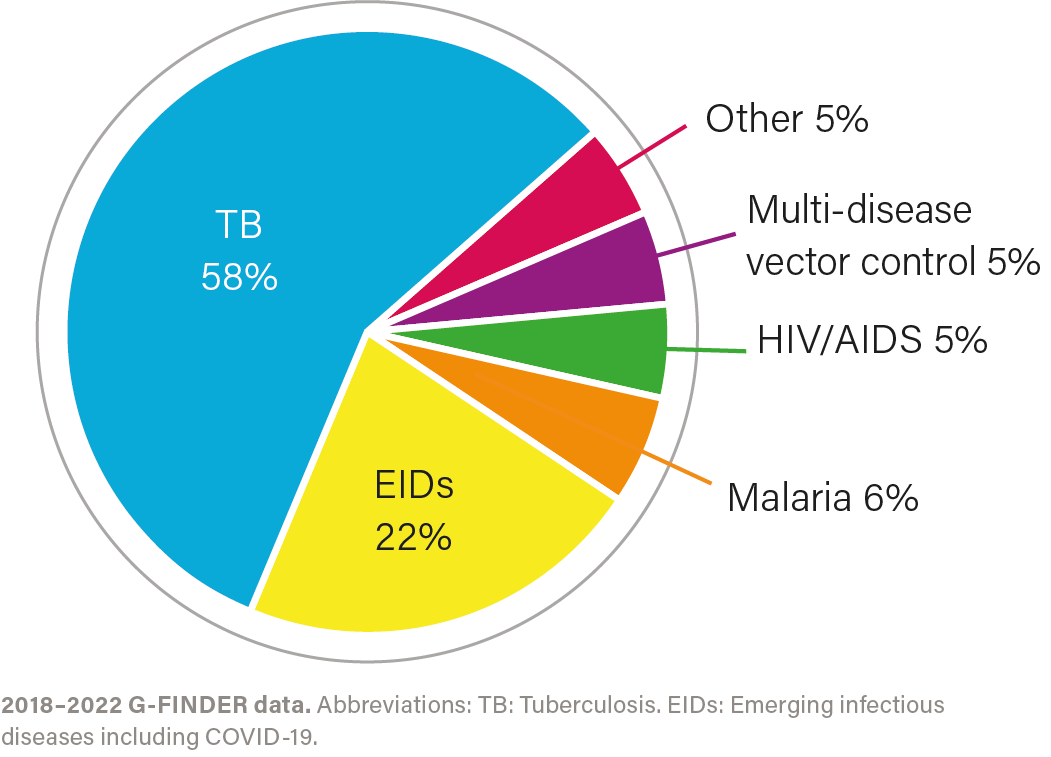
 R&D investment by health area
R&D investment by health area
IMPACT OF INVESTMENT
MALARIA
Evaluation of the efficacy of insecticide-treated bednets and the level of damage they can sustain before requiring replacement, which is increasing the impact of bednet programs while also reducing costs.
TB
Development of simplified, better-tolerated tuberculosis (TB) treatments through the Tuberculosis Trials Consortium, which have dramatically reduced TB treatment times and costs.
EBOLA
Development, evaluation, and distribution of rapid diagnostics for Ebola during the 2014–2015 outbreak, including a test that can provide results in as little as four minutes, as well as support for the clinical trials of the world’s first approved Ebola vaccine.
NTDs
Development of improved diagnostic tools for neglected tropical diseases (NTDs), including new tests for dengue, elephantiasis, river blindness, and schistosomiasis.
CAPACITY-STRENGTHENING
Training of more than 20,500 disease detectives in more than 80 countries through its flagship global Field Epidemiology Training Program.
COVID-19
Ongoing surveillance activities to detect and characterize new variants of the virus, including by leveraging cutting-edge advanced molecular detection capabilities.
Global Health R&D at the Biomedical Advanced Research and Development Authority
 What does BARDA do for global health R&D?
What does BARDA do for global health R&D?
The Biomedical Advanced Research and Development Authority (BARDA) supports the development of vaccines, drugs, and other medical countermeasures (MCMs) to protect Americans against threats to public health, including emerging infectious diseases, pandemic influenza, and antimicrobial resistance (AMR).
 Why is BARDA’s role in global health R&D important?
Why is BARDA’s role in global health R&D important?
BARDA works with industry and other partners to bridge the “valley of death” between basic research and product development, where research and development (R&D) efforts most often fail. Through unique contracting and incentive mechanisms, BARDA’s partnerships ensure promising research is translated into urgently needed medical products by creating commercial incentives for developers that would otherwise not exist. During the COVID-19 pandemic, BARDA’s prominence and funding grew exponentially as the agency was charged with leading the US government’s MCM R&D portfolio, demonstrating how—with sufficient, sustained funding—BARDA is uniquely equipped to advance products against a range of other global health threats.
 Impact of Investment
Impact of Investment
BARDA support has helped advance at least:
 R&D investment by health area
R&D investment by health area
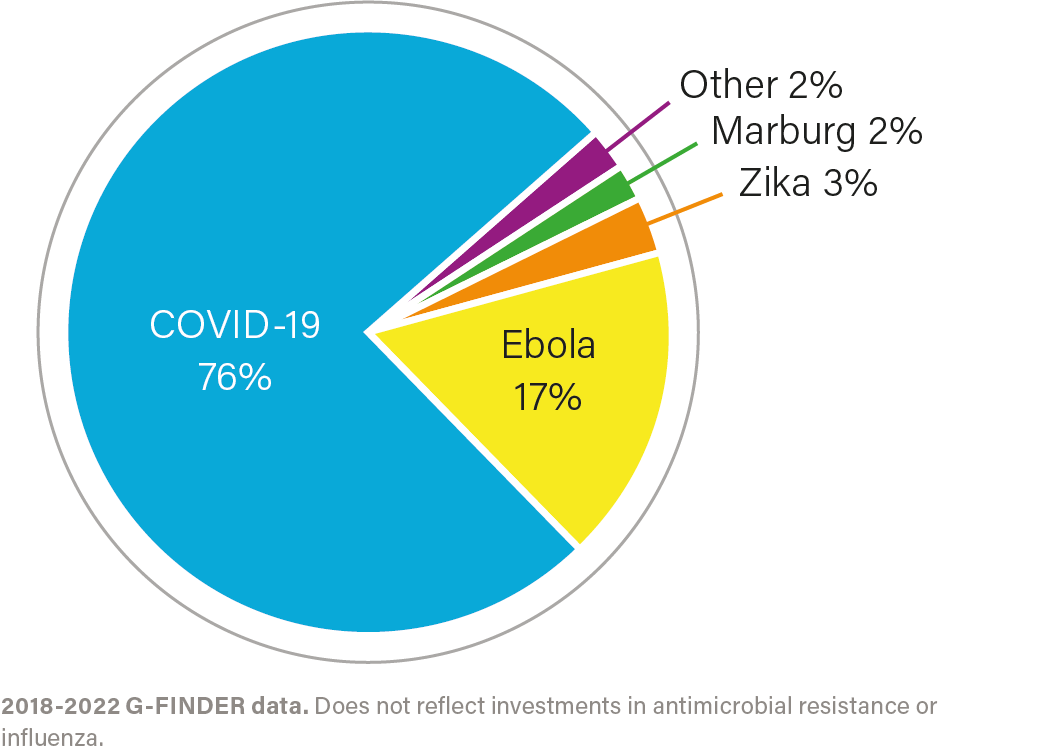
IMPACT OF INVESTMENT
COVID-19
Led US COVID-19 product development efforts for vaccines, diagnostics, and therapeutics, including advancing all therapeutics and first-generation vaccines approved or authorized by the US Food and Drug Administration (FDA).
EBOLA
Development of both Ebola Zaire vaccines, as well as two Ebola treatments and one rapid diagnostic test approved by FDA.
AMR
Accelerating antibacterial research through the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator, or CARB-X, public-private partnership, which has advanced 2 available diagnostics and supported 100 projects in 13 countries, to build the world’s largest early development pipeline of antibacterial innovations.
MPOX
Development of the only FDA-approved vaccine for smallpox/mpox, which was deployed during the 2022–2023 outbreak.
ZIKA
Development of Zika diagnostics, including tests to identify infection and screen blood supplies.
INFLUENZA
Strengthening manufacturing capacity in low- and middle-income countries to enable rapid production of seasonal and pandemic influenza vaccines.
Global Health R&D at the Department of Defense
 What does DoD do for global health R&D?
What does DoD do for global health R&D?
The Department of Defense (DoD) supports research and development (R&D) for infectious diseases, antimicrobial resistance, and other health conditions that pose a risk to US national security and service members stationed abroad.
 Why is DoD’s role in global health R&D important?
Why is DoD’s role in global health R&D important?
While DoD research first and foremost aims to protect service members while overseas, it also helps generate vaccines, drugs, and other health tools to combat diseases that are endemic in the world’s poorest places. Additionally, because DoD focuses on producing health tools for austere settings like the battlefield, the tools it advances are often well-suited for use in low-resource communities worldwide.
DoD research is unique in spanning across all stages of R&D, from basic research to late-stage clinical development and manufacturing, making it the only US agency that can single-handedly advance a single technology from early research to end-stage product.
 Impact of investment
Impact of investment
DoD support has helped advance at least:
 R&D investment by health area
R&D investment by health area
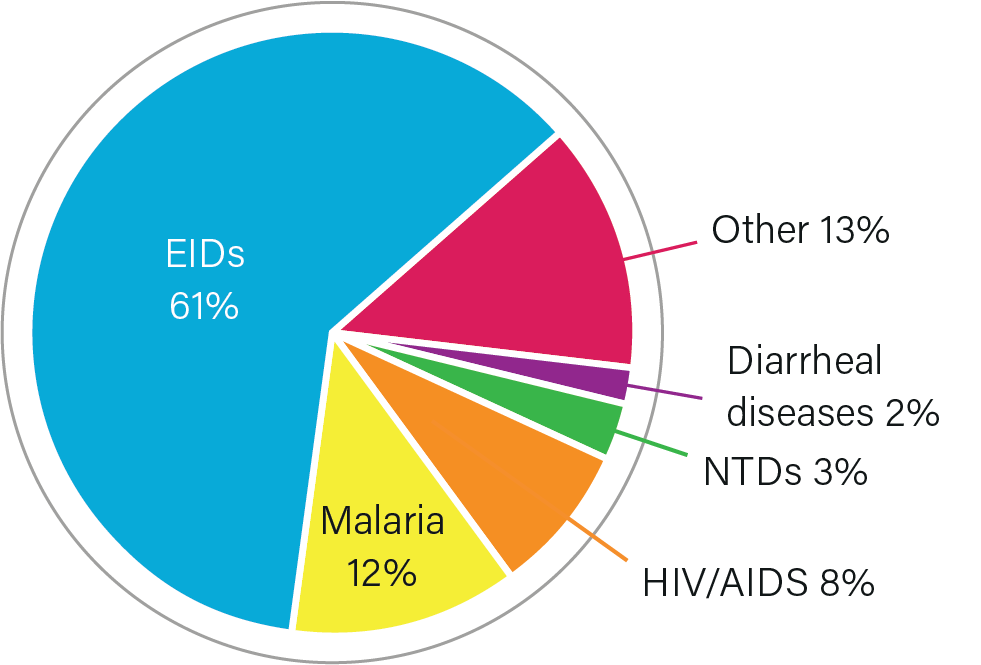
IMPACT OF INVESTMENT
MALARIA
Development of nearly every antimalarial drug approved by the US Food and Drug Administration and the world’s first approved malaria vaccine, which has been given to more than 1.7 million children in sub-Saharan Africa.
HIV/AIDS
Supporting ongoing development of potential HIV/AIDS vaccine regimens, including a trial in Uganda that combines experimental vaccines, an Army-developed adjuvant, and a novel dose escalation approach in hopes of boosting immune response.
DIARRHEAL DISEASES
Ongoing development of affordable vaccines against Escherichia Coli and Shigella, and creation of the technology used in a water chlorinator device, which gives low-resource communities access to safe, affordable drinking water.
EBOLA
Development of both Ebola Zaire vaccines, which have been used to successfully quell recent outbreaks of the disease, and a rapid, automated diagnostic test.
COVID-19
Development of rapid diagnostics for COVID-19 and the Novavax COVID-19 vaccine and supporting ongoing development of a pan-coronavirus vaccine to protect against multiple COVID-19 variants and other coronaviruses.
CAPACITY-STRENGTHENING
Operates a network of overseas labs and medical research facilities, which provide bases for infectious disease R&D, disease surveillance, and capacity-strengthening in partner nations.
Global Health R&D at the US Food and Drug Administration
 What does FDA do for global health R&D?
What does FDA do for global health R&D?
The US Food and Drug Administration (FDA) regulates the safety and efficacy of drugs, vaccines, and other medical products marketed in the United States, which can include products also designed for use overseas. FDA also works with the World Health Organization (WHO) and international regulators in low- and middle-income countries (LMICs) to strengthen regulatory capacity and provide technical assistance.
 Why is FDA ’s role in global health R&D important?
Why is FDA ’s role in global health R&D important?
FDA approval of a product serves as a “gold standard” that can expedite regulatory review in LMICs. This effect, combined with the agency’s work in regulatory capacity-strengthening, helps ensure new global health technologies are safe, effective, and accessible in low-resource settings.
 Ensuring safe & effective products
Ensuring safe & effective products
FDA has approved more than 110 drugs, vaccines, and diagnostics for neglected and emerging diseases.
FDA has 208 formal arrangements for information sharing and technical assistance with regulatory authorities in 55 countries.
Contributions to global health R&D
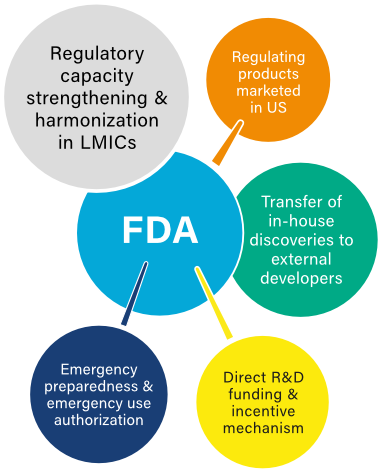
IMPACT OF INVESTMENT
CAPACITY-STRENGTHENING
Development of partnerships to strengthen regulatory capacity in LMICs, including collaborations with the WHO African Vaccine Regulatory Forum to share expertise and provide training and mentoring.
HIV/AIDS
Creation of a “tentative approval” process to certify antiretroviral (ARV) drugs that the President’s Emergency Plan for AIDS Relief, or PEPFAR, purchases for use outside the United States. Through the program and standard review, FDA has approved more than 250 ARVs, which have helped support treatment for more than 20.5 million people worldwide via PEPFAR.
HEALTH EMERGENCIES
Granted emergency use authorization for more than 400 medical products for COVID-19, 9 products for mpox, 18 diagnostics for Zika, and 11 diagnostics for Ebola, facilitating their use during these crises.
MENINGITIS
Development of critical technology used in a low-cost meningitis A vaccine, which as of 2020, has been delivered to more than 340 million people in 24 countries, virtually eliminating meningitis A wherever it has been used.
NTDs
Release of guidance documents to aid organizations in developing drugs for neglected tropical diseases (NTDs) and issuance of 14 priority review vouchers (PRVs) as part of the NTD PRV program intended to stimulate private-sector investment in NTD R&D.
TB
Use of an accelerated approval pathway to speed review and approval of two new drugs to treat drug-resistant tuberculosis (TB).
